RNA sequencing has become an indispensable tool for researchers interested in studying transcriptome-wide analysis of differential gene expression and differential splicing of mRNAs. As next-generation sequencing technologies have developed, so too has RNA-seq. The ability to study the transcriptome has been a vital tool for many areas of biology, including cancer research and therapy, biomarker discover, drug resistance, immunotherapy, neoantigens, and developmental biology, to name a few.
MedGenome offers RNA-seq solutions for studying different aspects of RNA biology, including both bulk and single cell solutions. Kits are available to fit the needs of most projects, including kits that accommodate extremely low input and low quality RNA. Our available assays allow for flexibility when designing RNA-seq experiments, make it possible to generate data from difficult-to-process samples, and keep costs low to allow for larger experiments. Here are a few things to consider when choosing the right RNA-Seq assay and kit for your experiment.
RNA-Seq technology provides scientists with a window into how cells and tissues function by measuring levels of gene expression. Since all normal cells within an organism have the same genome, differences in cell identities and function can be determined by analyzing gene expression. Bulk RNA-Seq experiments provide a view of gene expression of an entire sample. This provides insight into the overall gene expression patterns in a population of cells, and allows researchers to compare gene expression patterns in different groups of samples (treated vs untreated, for example). Bulk RNA-Seq is a powerful tool for researchers since it is inexpensive and can be used to process a wide range of samples, including degraded RNA. However, bulk RNA-Seq does not differentiate among cell types within the sample, rather they give a view of gene expression within a whole organ or tissue type. Single cell RNA-Seq technology allows for the study of gene expression in individual cells in a heterogeneous population. More information on single-cell RNA-Seq can be found here.
The RNA-Seq process is based around the construction of complementary DNA (cDNA) library to be used for sequencing. Library construction begins with the isolation of RNA, followed by quality control measures to determine quantity and quality of the RNA. After this, a depletion or selection strategy is chosen to enrich the library of the RNA species of interest. RNA is then reverse-transcribed into cDNA before being converted into a sequencing library. Whether total RNA or mRNA sequencing should be used is determined by the objective of the experiment and the types of samples being studied, as several important differences exist between these RNA-seq methods. Total RNA sequencing is the most comprehensive approach and typically involves sequencing both coding and non-coding RNA molecules. Generally, the RNA has been depleted of ribosomal RNA (rRNA), which represents the majority of RNA molecules. The resulting sequencing data will include mRNA, pre-mRNA, and long non-coding RNA (data will not include microRNA). An additional benefit with these kits is that they can generally work with highly degraded RNA samples. Since probes rRNA must be identified and depleted, these kits are often species-specific. However, if the research goal is to focus primarily on the coding region, then mRNA sequencing is the best choice. These protocols use a selection method to enrich for polyadenylated (poly(A)) RNA. Since mRNA represents only a small percentage of the total RNA molecules, sequencing only mRNA is the most cost-effective and efficient method if it meets the overall experimental goals. mRNA sequencing kits do require higher quality RNA as input.
| Kit | Assay Type | Stranded Type | Starting Material | Input Amount |
|---|---|---|---|---|
| TruSeq Stranded mRNA | mRNA Seq | Yes | RNA | 100 ng - 1 μg RNA |
| Illumina Stranded mRNA | mRNA Seq | Yes | RNA | 25 ng - 1 μg RNA |
| Takara SMART-Seq V4 | mRNA Seq | No | RNA, Cells | 10 pg - 10 ng RNA, 1 - 1,000 cells |
| TruSeq Stranded Total RNA | Total RNA | Yes | RNA | 100 ng - 1 μg RNA |
| Pico V2 / V3 | Total RNA | Yes | RNA | 250 pg - 10 ng RNA |
| SMART-Seq Stranded | Total RNA | Yes | RNA, Cells | 10 pg - 10 ng RNA, 1 - 1,000 cells |
TruSeq Stranded mRNA (Illumina) is considered the gold standard by many. This kit allows for high input (0.1 to 1 μg) of RNA to generate mRNA libraries. This protocol uses oligo(dT) beads to capture polyadenylated mRNA. Additionally, dUTP is used during second strand cDNA synthesis to quench this strand during PCR amplification, resulting in a library that represents only the first strand cDNA information.
Stranded mRNA Prep is Illumina’s latest kit for sequencing mRNA. It uses similar chemistry to TruSeq Stranded mRNA to capture and reverse transcribe polyadenylated mRNA, however the library preparation has been upgraded to ligate anchors for enrichment. This kit uses 25 ng to 1 μg RNA as input to generate strand-specific data. The lower input requirement can be ideal when RNA quantity is limited.
SMART-Seq v4 (Takara) is a low input mRNA sequencing kit. High quality data can be generated using RNA as input (10 pg – 10 ng) or using cells as direct input. Although this kit does not generate strand-specific data, the ability to analyze extremely low quantities of RNA makes it an excellent choice for mRNA sequencing. After generating amplified cDNA using the SMART-Seq v4 kit, Illumina’s Nextera XT DNA Library Preparation kit is then used to generate the library.
TruSeq Stranded Total RNA (Illumina) allows for robust interrogation of both standard and low-quality samples, and is compatible with a wide range of studies. This kit relies on probes to deplete rRNA before generating a stranded sequencing library. The kit allows for high RNA input (100 ng to 1 μg) which increases complexity of the library. Additionally, there are variations of this kit available for sequencing blood and bacteria samples.
SMARTer Stranded Total RNA-Seq Kit v2/v3 (Pico v2/v3) is designed for efficient preparation of sequencing libraries from picogram amounts (250 pg – 10 ng) of RNA. This kit works extremely well with degraded RNA, including RNA extracted from FFPE samples. In this protocol, rRNA is enzymatically digested using mammalian-specific probes. Additionally, the new version 3 kit includes UMIs to reduce potential PCR-bias in the data.
The SMART-Seq Stranded kit from Takara is another option for low-input total RNA sequencing. This kit uses similar chemistry to Takara’s Pico v2 kit, however it can be used with even lower input (10 pg – 10 ng RNA). It also offers the option to use cells as direct input (1 – 1,000 cells). This kit also uses mammalian-specific probes and is a great option when you have limited quantity of cells or RNA but want Total RNA-seq data.
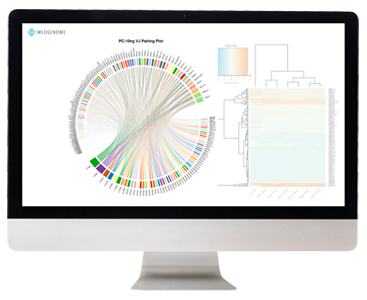
Our data analysis starts with the generation of a Multi-QC Report. This is a modular tool to aggregate results from bioinformatics analyses across many samples. This report provides deep insight on overall data QC, alignment statistics, and a FastQC report. These data represent the overall success of the library prep and sequencing.
Reads are aligned to the transcriptome and expression values are reported in FPKM (Fragment Per Kilobase per Million). Groups of samples can then be compared to detect differentially expressed genes. We generate publication-ready plots, such as heat maps and volcano plots, to visualize significant trends in the data. We also offer pathway analysis and tumor microenvironment analysis for deeper understanding of these data.
