By Dr. Lavanya Balakrishnan and Vinay C. G., MedGenome Scientific Affairs
Illumina’s TSO 500 empowers comprehensive genomic profiling (CGP), unlocking crucial tumor biomarkers to drive precision medicine. By focusing on clinically relevant genomic regions, CGP provides in-depth analysis, accurately detects low-frequency mutations, and comprehensively characterizes tumors. This facilitates tailored treatment approaches, guiding therapy selection based on specific molecular profiles. Moreover, CGP enables non-invasive disease monitoring through circulating tumor DNA (ctDNA) analysis.
TSO 500: Advanced NGS assay for in-depth pan-cancer genomic profiling
TSO 500 is a cutting-edge next-generation sequencing (NGS) assay designed for pan-cancer genomic profiling. TSO 500 targets the full coding regions of 523 genes known to be implicated in cancer and offers a comprehensive analysis of genetic alterations in solid tumors including single nucleotide variants (SNVs), insertions/deletions (InDels), copy number variations (CNVs), gene fusions and splice variants. Furthermore, TSO 500 also assesses microsatellite instability (MSI) and tumor mutational burden (TMB), biomarkers crucial for understanding the tumor’s behavior and potential response to immunotherapy1.
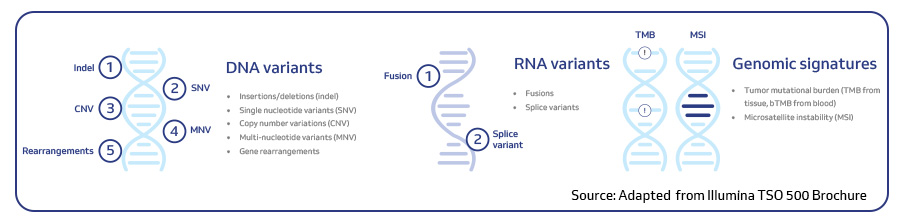
TSO 500 employs a hybridization-based capture approach using unique molecular identifiers (UMIs) and allows the analysis of both DNA and RNA from a single sample1. The TSO 500 portfolio offered by MedGenome includes two assays: TSO 500 for tissue-based profiling and TSO 500 ctDNA for liquid biopsy analysis.
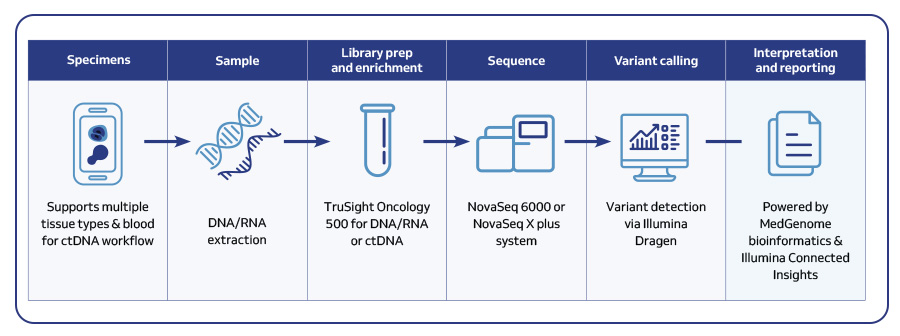
| TSO 500 | TSO 500 ctDNA | |
|---|---|---|
| Sample type | Tissue biopsies and FFPE | Blood |
| Sample input and amount | DNA and RNA; 50 ng purified DNA and RNA | Cell free DNA; 20–30 ng purified ctDNA |
| Genes covered | 523 genes for DNA variants; 55 genes for RNA fusions and splice variants | 523 genes for DNA variants; 23 genes for DNA fusions |
| Variants called and Genomic signatures |
|
|
| Panel size | 1.94 Mb DNA; 358 kb RNA | 1.94 Mb DNA |
| Sequencing platform and read depth | Novaseq 6000 or Novaseq X plus DNA: >80 M PE Reads; 100 bp PE RNA: >40 M PE Reads; 100 bp PE | Novaseq 6000 or Novaseq X plus >400 M PE Reads; 150 bp PE |
| Approximate turnaround time | 5-7 days | 5-7 days |
| Sample batching | TSO 500 – 8 samples/run TSO 500 HRD – 8-16 samples/run | 8–48 samples/run |
| Analytical specificity | 99.9998% | 99.9994% (SNVs) |
| Analytical sensitivity | >96% | >95% |
Predictive biomarker identification: TMB and MSI with TSO 500
The TSO 500 assay is a comprehensive solution for identifying patients likely to benefit from immunotherapy. By accurately quantifying TMB and determining MSI status, two key predictive biomarkers, the assay allows to make informed treatment decisions. Leveraging error-corrected sequencing and robust bioinformatics, the assay enables precise measurement of TMB, including both synonymous and nonsynonymous mutations, and reliable assessment of MSI. Both TMB and MSI values generated by this assay demonstrated high concordance with those obtained from whole exome sequencing and PCR-based assays, respectively.
From data to insights: Illumina Connected Insights and Dragen
Illumina Connected Insights, powered by the Dragen bioinformatics platform, transforms complex genomic data into actionable insights. By integrating the TSO 500 assay, this powerful combination delivers comprehensive tumor profiling, enabling precise patient selection and optimized treatment strategies. Dragen rapidly processes vast amounts of sequencing data, providing accurate variant calls, while Illumina Connected Insights offers a user-friendly interface for interpretation, clinical decision support, and seamless workflow integration.
Effectiveness of TSO 500 in cancer immunotherapy
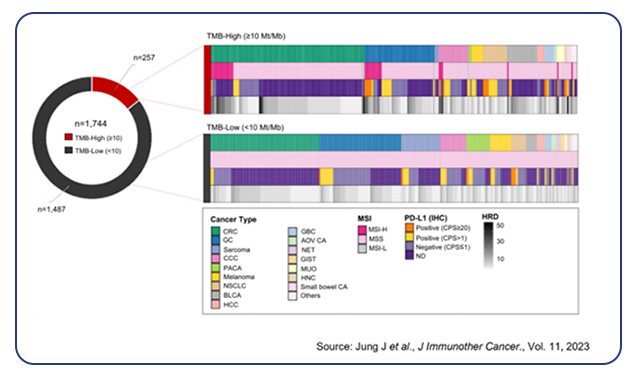
The TSO 500 assay has proven highly effective in immunotherapy by identifying biomarkers such as TMB and MSI that predict response to immune checkpoint inhibitors. Several studies have shown how the assay has helped identify actionable mutations in various types of cancer, leading to successful targeted therapies. Validation of TSO 500 on 170 clinical samples across different cancers demonstrated precision and accuracy of over 99%, with sensitivity and specificity of at least 99% for all variant types2. Using this assay, higher response rates to anti-PD-(L)1 therapy in TMB-high cases were observed, particularly in gastric, gallbladder, head and neck cancers, and melanoma3. It also highlighted significant genetic alterations, such as BRAF mutations in peritoneal metastases from colorectal cancer4 and heterogeneity in intrahepatic cholangiocarcinoma5. Additionally, TSO 500’s comprehensive profiling of early-stage NSCLC6 and endometrial serous carcinoma7 revealed actionable mutations and potential markers for prognosis and treatment stratification. These findings underscore TSO 500’s role in enhancing diagnostic accuracy and guiding therapeutic decisions across different cancer types.
How MedGenome’s comprehensive genomic profiling enhances targeted therapies
MedGenome offers end-to-end customized genomic profiling solutions to accelerate cancer research. Our expertise in handling diverse sample types, including tumor biopsies, FFPE tissues, and liquid biopsies, combined with advanced bioinformatics capabilities, ensures rapid and accurate analysis. With rapid turnaround times and scalable operations, MedGenome empowers researchers to unlock the potential of precision oncology. We analyze TSO 500 data using Dragen with Illumina Connected Insights, delivering the highest accuracy in variant calls with the fastest analysis time. Our variant summary report includes results from Dragen and Illumina Connected Insights, with advanced analysis reports featuring rich visualizations of mutations, fusions, copy number alterations, and immuno-oncology biomarkers such as TMB and MSI.
Contact us now to learn how our TSO Targeted sequencing can drive your research forward.
References
-
- https://sapac.illumina.com/products/by-brand/trusight-oncology/tso-500-portfolio.html
- Froyen G, Geerdens E, Berden S, Cruys B, Maes B. Diagnostic Validation of a Comprehensive Targeted Panel for Broad Mutational and Biomarker Analysis in Solid Tumors. Cancers (Basel), 14, 2457 (2022).
- Jung J, Heo YJ, Park S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: a real-world pan-tumor analysis. J Immunother Cancer., 11, e006454 (2023).
- Heuvelings DJI, Wintjens AGWE, Moonen L, Engelen SME, de Hingh IHJT, et al. Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer. Int J Mol Sci., 24, 12830 (2023).
- Kinzler MN, Schulze F, Jeroch J, Schmitt C, Ebner S, et al. Heterogeneity of small duct- and large duct-type intrahepatic cholangiocarcinoma. Histopathology, 84, 1061-1067 (2024).
- Choi SJ, Lee JB, Kim JH, Hong MH, Cho BC, Lim SM. Analysis of tumor mutational burden and mutational landscape comparing whole-exome sequencing and comprehensive genomic profiling in patients with resectable early-stage non-small-cell lung cancer. Ther Adv Med Oncol., 16, 17588359241240657 (2024).
- Aisagbonhi O, Ghlichloo I, Hong DS, Roma A, Fadare O, et al. Comprehensive next-generation sequencing identifies novel putative pathogenic or likely pathogenic germline variants in patients with concurrent tubo-ovarian and endometrial serous and endometrioid carcinomas or precursors. Gynecol Oncol., 187, 241-248 (2024).
#Tumor profiling, #Next-generation sequencing, #Targeted sequencing, #circulating tumor DNA, #ctDNA, #TSO 500, #TSO 500 ctDNA, #Tumor mutational burden (TMB), #Microsatellite instability (MSI), #Liquid biopsies, #Error corrected sequencing, #Illumina Connected Insights, #Dragen, #Precision oncology, #Targeted therapies
 US
US IN
IN

