By Vinay CG, Derek Vargas and Kushal Suryamohan, MedGenome Scientific Affairs
According to the American Cancer Society, an estimated 1.9 million new cancers will be diagnosed in 2022 [1]. Some of the major cancer types affecting the population are prostate, lung & bronchus, colon & rectum, urinary bladder, melanoma of the skin, kidney & renal pelvis, non-Hodgkin lymphoma, oral cavity & pharynx, leukemia, pancreas, breast, colon & rectum, uterine corpus, thyroid. Lung and Bronchus (21%) in both men and women, prostate in men (11%) and breast cancer (31%) in women are the majority cancer types causing death in the population [1]. Even though our understanding of cancer has broadened over the years it is still a major challenge to tackle across the globe. Widely accepted therapy forms for cancer includes biomarker identification and testing for treatment, chemotherapy, hormone therapy, immunotherapy, photodynamic therapy, radiation therapy, stem cell transplant, surgery, and targeted therapy. Immunotherapy (Table 1) is emerging as a forerunner among all the types of cancer therapies for the simple reason as it considers the various dynamics of immune function in an individual. Genomics has played a key role in enabling the identification of therapeutically actionable targets and in guiding the use of immunotherapy.
The magic of immunotherapy – proven right again
Recently, a team of doctors at Memorial Sloan Kettering Cancer Center published the results of a cancer trial in the New England Journal of Medicine involving Dostarlimab – a potential immunotherapy drug – to be effective in a small group of patients of 14 suffering from rectal cancer who went into complete remission [2,3]. Dostarlimab belongs to a class of drugs called checkpoint inhibitors – a programmed death 1 (PD-1) blockade drug. Mismatch Repair (MMR)-deficient colorectal cancer was found to respond well to PD-1 blockade in this trial and hence the success. PD-1 prevents T cells from killing cancer cells and thus by blocking PD-1, it is possible to activate the T-cell machinery that can then effectively kill the cancerous cells.
Mismatch repair-deficient (MMRd) or Microsatellite instability (MSI) [4] are additional factors that are linked with higher chance of developing cancer. This MMR deficiency is common in colorectal, gastrointestinal, and endometrial cancers. Finding the tumor cells with MMR deficiency can be very useful in determining the course of the treatment. The presence of MSI has also been identified as a predictor of a response to immune-checkpoint inhibition, leading the FDA to approve the anti-programmed cell death protein 1 (PD-1)-antibody pembrolizumab, for use in patients with MSI-high solid tumours regardless of histology or anatomical location.
Table 1: Types of Immunotherapies [5]
| Immunotherapy Type | Mode of Action |
|---|---|
| Monoclonal Antibodies (mAbs) | They are the special kind of proteins which are designed to target antigens or markers present on cancer cells. |
| Check Point Inhibitor Drugs | These drugs’ common targets are CTLA-4 and PD-1/PD-L1. The check point inhibitor drugs release the breaks allowing T cells to attack the cancer cells more efficiently. |
| Cancer Vaccines | They trigger an immune response by identifying and attacking certain marker or antigens present on the cancer cells |
| Oncolytic Virus Immunotherapy | Oncolytic Viruses are the genetically modified viruses that can attack the cancer cells directly. They are often combined with other types of immunotherapies such as a cancer vaccine / mAb therapy. |
| Adoptive T Cell Transfer | It is an anti-cancer approach where the immune cells are made effective to tackle cancer. One such special approach is to add Chimeric Antigen Receptors (CARs) to T cells in the lab and reinfuse to patient. CAR T cells then can identify cancer cells and kill them. |
| Cytokines | They aid in control and growth of immune cells |
| Adjuvant Immunotherapy | These involve methodologies where ligands are used to boost immune response |
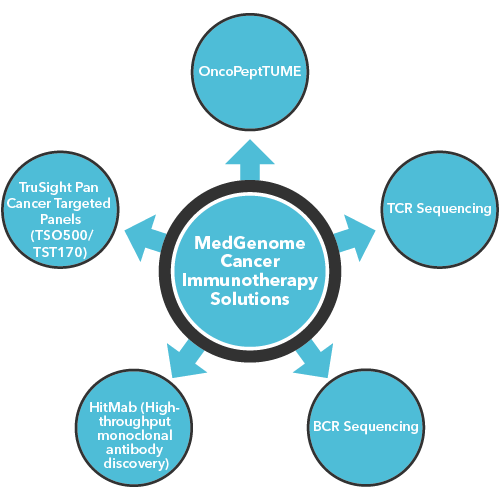
How MedGenome’s sequencing solutions are highly effective in supporting immunotherapy?
MedGenome has a primary focus on tackling immunotherapy challenges through various types of sequencing solutions:
-
- OncoPeptTUME: It is a proprietary platform which interrogates RNA-Seq data sets to produce high resolution mapping of the tumor microenvironment using proprietary cell type specific gene expression signatures. It can be customized to fit cancer immunotherapy project needs and tailored to perform in preclinical and clinical settings.
-
- TCR Sequencing: With the aid of Next-Generation Sequencing we offer deeper insights such as
CDR3 repertoire diversity, clonal composition, potential antigenic recognition spectrum, and the quantity of antigen specific T-cell responses – that can be very useful in prescribing the right Immunotherapy for the patients.
- TCR Sequencing: With the aid of Next-Generation Sequencing we offer deeper insights such as
-
- BCR Sequencing: Our BCR sequencing solutions offer wider insights such as B-cell differentiation, BCR somatic hypermutation, class switching, and antigen specificity.
-
- HitMab (High-throughput monoclonal antibody discovery): MedGenome’s HitMab platform accelerates drug discovery process through its Single Cell BCR sequencing methodologies that provide crucial information on heavy and light chain antibodies with greater specificity, enables antibody generation for low or poorly immunogenic proteins and offers distinct advantages over the current Hybridoma technology.
-
- TruSight Pan Cancer Targeted Panels (TSO500/TST170): Offers a distinct advantage in identifying immuno-oncology biomarkers such as microsatellite instability (MSI) and tumor mutational burden (TMB). It also helps in assessing fusions, splice variants, insertions/deletions and single-nucleotide variants (SNVs), and amplifications.
Our recent efforts on identifying novel neoantigens in Gallbladder Cancer published in Nature [6]:
MedGenome was part of a multi-collaborative study aimed at identifying key actionable targets and identify potential immunotherapy strategies to treat gallbladder cancers (GBC). The genomic analysis of 167 gall bladder cancer samples revealed mutated GBC genes that include several targetable driver genes such as ERBB2, ERBB3, KRAS, PIK3CA, and BRAF. Since there is no approved line of immunotherapy treatment for Gall Bladder cancers, our efforts successfully identified neoantigens from several mutated GBC genes including ELF3, ERBB2, and TP53. Validation in the lab showed T-cell activation thus indicating that they are potential cancer vaccine candidates. Additionally, some of the samples from this study had MSI which could also be targets for checkpoint inhibitor therapy.
Want to know more about our unique Cancer Immunotherapy Solutions?
Get in touch with our experienced and seasoned scientific team to understand how our unique cancer immunotherapy solutions can provide deeper insights to your research projects. You can also email us at research@medgenome.com for any queries and further details.
References
- 1. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- 3. https://www.theguardian.com/science/2022/jun/08/rectal-cancer-research-breakthrough-experimental-treatment-remission
- 4. https://www.mskcc.org/news/rectal-cancer-disappears-after-experimental-use-immunotherapy
- 5. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mismatch-repair-deficiency
- 5. https://www.cancerresearch.org/en-us/immunotherapy/what-is-immunotherapy
- 6. Pandey, A., Stawiski, E.W., Durinck, S. et al. Integrated genomic analysis reveals mutated ELF3 as a potential gallbladder cancer vaccine candidate. Nat Commun 11, 4225 (2020). https://doi.org/10.1038/s41467-020-17880-4
#Microsatellite instability (MSI), #MMR deficiency, #Immunotherapy, #CancerImmunotherapy, #Dostarlimab, #Gall Bladder cancer, #tumor mutational burden, #TCR Sequencing, #BCR Sequencing, #Checkpoint Inhibitor, #Monoclonal Antibodies, #HitMab, #Oncolytic Virus Immunotherapy, #Adoptive T Cell Transfer, #PD-1, #cancervaccine, #RNA Seq
 US
US IN
IN

