By Dr. Anantha Kethireddy Ph. D., MedGenome Scientific Affairs
Why is Alzheimer’s relevant?
Alzheimer’s disease (AD) has long been one of the great challenges in medicine and imposes a constant burden on our aging population. Recent statistics show that approximately 50 million people worldwide suffer from AD or some other form of dementia. The World Health Organization has estimated that the total number of people with dementia worldwide will reach 82 million by 2030 and 152 million by 2050. Of the top 10 leading causes of death based on United States cancer statistics, cardiovascular disease ranks first, tumors rank second and AD ranks sixth.
AD is a slowly progressing and eventually fatal neurodegenerative disorder and a major contributor of dementia leading to degeneration of neurons and their connections in parts of the brain involved in memory. The symptoms are impairment in thinking, remembering, reasoning, cognitive functions and behavior are known as dementia. Other diseases and conditions can also cause dementia, with AD being the most common cause of dementia in older adults. AD is not a normal part of aging. It’s the result of complex changes in the brain that starts years before symptoms appear and lead to loss of brain cells and connections. The hallmark of AD is the presence of plaques of the amyloid and neurofibrillary tangles of the phosphorylated protein tau. Much evidence suggests the involvement of neuroinflammation, multiple systemic comorbidities in the pathology of AD.
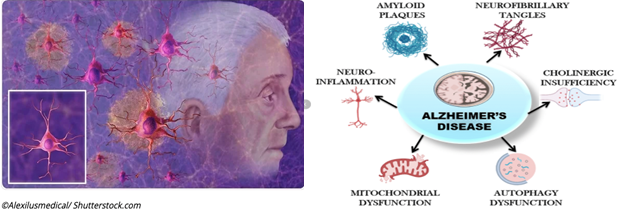
Since AD was first described in the early 1900s, clinicians and scientists all over the world dedicated their career to study the pathophysiology of this most common form of disease in the hope of developing methods of prevention, treatments to halt the progression and ultimately a cure. AD’s pathophysiology involves neuron-glia interactions, supported by transcriptomic and epigenomic analyses that reveal downregulation of neuronal functions and upregulation of innate immune responses in AD brains. Currently, there are some FDA approved tools that, when applicable, can be used to aid in diagnosis of AD symptoms (brain imaging), while other emerging biomarkers are promising but still under investigation (blood tests, genetic risk profiling).
Drugs do exist, if administered early enough they may help to treat the symptoms of early-stage AD and improve the person’s quality of life. Very recently, two pharmacological companies announced encouraging results from a clinical trial for patients with AD. A monoclonal antibody treatment, called lecanemab, suppressed cognitive decline by 27% in people with early-stage disease compared with those on a placebo after a year and half. Black or Hispanic populations also have a higher risk of Alzheimer’s disease than non-Hispanic white people, researchers don’t fully understand the reasons. There is no drug that works in everybody to stop or reverse AD.
Understanding AD
The molecular and cellular mechanisms of AD is incompletely understood. Typically, when scientists studied gene expression in the brain, they just mashed up the tissue and took average measurements from that mixture. Such “bulk” measurements are hard to interpret, and we lose the gene expression signals that come from individual cell types especially for lowly-represented cell types. Although numerous studies using bulk RNA-seq analysis have revealed dysfunctions of neurons and/or innate immune responses, they are unable to entangle the heterogeneity of different disease subtypes and distinct responses across cell types. Characterization of the heterogeneity of a region of the brain important for learning and memory, the first region affected in Alzheimer’s disease.
Single Cell Genomics
Even within a single brain region, there is a significant variation between the morphology, connectivity and electrophysical properties of individual neurons. A key step towards understanding the basic components of the nervous system is systematic classification of individual neurons. For cells to be classified on a molecular basis, gene expression must be assessed at single-cell resolution. Today’s high throughput technologies such as single cell and spatial multiomics are revolutionizing neurological research at single cell level resolution.
A cohesive demonstration of how gene expression is regulated within discrete cell types and specific anatomical regions of the brain during the early stages of AD is crucial to study the cellular heterogeneity of the brain by profiling tens of thousands of individual cells, capturing the molecular and cellular basis of AD and identifying novel therapeutic targets.
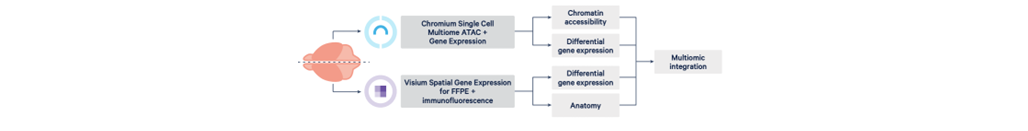
Figure 2: Multiomic integration from single brain (Image source: 10x Genomics)
Summary
Capturing a holistic view of cell type specific contributions to pathogenesis, mapping anatomical protein accumulation in the brain during disease progression, and understanding the relationship between abnormal protein accumulation and cellular phenotypes diagnosis at an early stage is very crucial to save the people from this dreadful disease.
By using Chromium Single Cell Multiome ATAC (Assay for Transposase-Accessible Chromatin) + Gene Expression (the multiome assay), which profiles open chromatin and gene expression from the same cell, and Visium Spatial Gene Expression for FFPE (Visium for FFPE) plus immunofluorescence (IF), which combines whole transcriptome spatial analysis with immunofluorescence protein detection will help to extract the shared and unique neurological disorders among different conditions.
The multidimensional datasets obtained through single cell genomics approaches will have major impact on biological research and clinical pathology. implementation and expansion of single cell technologies will lead to vast improvements in the diagnosis and treatment of patients worldwide.
References
- 1. www.alzheimers.gov
- 2. Liu-Lin Xiong et al., Single-cell RNA sequencing reveals B cell–related molecular biomarkers for Alzheimer’s disease, Nature, 2021
- 3. Application note: Single cell and spatial multiomics identifies Alzheimer’s disease markers, 10xgenomics.com/rs/446-PBO-704/images/10x_App-Note_Alzheimers_Letter_Digital.pdf
- 4. Science insider, https://www.science.org/content/article/five-big-questions-about-new-alzheimer-s-treatment
- 5. National Institute of Aging: Alzheimer’s disease fact sheet
- 6. Vinay C.G, Project report on Alzheimer’s disease and its treatment. Nano Science and technology consortium, 2013
Connect with MedGenome, your 10X certified single-cell sequencing expert, to drive your research breakthroughs.
#Spatial Multiomics, #Single Cell, #Alzheimer’s disease, #Single Cell Genomics, #Single Cell Multiome ATAC , #whole transcriptome spatial analysis
 US
US IN
IN

