By Dr. Anantha Kethireddy Ph. D., MedGenome Scientific Affairs
Modern medicine now derives its insights through the deeper understanding of the cellular and molecular mechanisms, which involves modification of the cellular behavior through targeted molecular approaches. Experimental biologists and clinicians now employ various molecular techniques to assess the intrinsic behavior of cells in a variety of ways, such as through analyses of genomic DNA sequences, chromatin structure, messenger RNA (mRNA) sequences, non-protein-coding RNA, protein expression, protein modifications and metabolites.
Today, single‐cell RNA expression profiling is rapidly becoming an irreplaceable method for various research including humans, animals and plants enabling more accurate, rapid identification of rare and novel cells in tissues like never before (Figure 1). Moreover, with this information about gene expression at mRNA and protein levels, metabolites, cell‐cell communication, and spatial landscape, it becomes possible to solve the puzzle of cell composition and functions in health and disease.
Although single cell sequencing studies have been conducted mostly by research groups over the past few years, it has become clear that biomedical researchers and clinicians can make important new discoveries using this powerful approach. While great promises have been demonstrated with the technological advancement in all areas, and its great potentials in transforming current protocols in diagnosis of the genetic drivers of the disease and treatment response mechanism from single cells to tissues.
Currently, there is a growing demand for single-cell technology, with nearly 200 different methods to profile not only transcriptomic but genetic, epigenetic, and proteomic information in individual cells.
An Overview of Single–Cell Technologies:
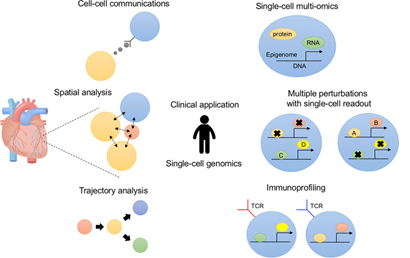
Single–cell technologies can be broadly classified into analysis of either DNA (genomics, epigenomics) or RNA (transcriptomics) with newer applications around the corner moving to combine both within the same cell.
Single-Cell genomics
Of relevance to cancer biology, is the ability to study genetic variations in individual cells. Although bulk DNA sequencing (DNA-seq) can be used to infer clonal sub-populations based on variant allele frequency analysis, it cannot be used to definitively test the co-occurrence of specific mutations in individual cells. Thus, single-cell DNA sequencing (scDNA-seq) can reveal cancer clonal architecture in far greater detail.
Single-Cell Transcriptomics
Single-cell transcriptomics has been used to study cancer stem cells, metastasis-initiating cells, chemotherapy resistance, and cancer immune responses.
Single-Cell Epigenomics
Many epigenetic processes (including DNA methylation, histone modifications, and chromatin accessibility) become dysregulated in cancer, and this fact has been exploited in various clinical applications. Single cell epigenomics can reveal the regulatory processes that lead to transcriptional heterogeneity in cancer, with important clinical implications. Single-cell DNA methylation analysis has also been applied to characterize circulating tumor cells and response to epigenetic therapies.
Single-Cell multiomics
It is also possible to combine analysis of the genome, transcriptome, epigenome, and other modalities using single-cell multiomic analyses. These combinatorial approaches allow genetic regulation to be studied in incredible detail and were named the 2019 “Method of the Year” by Nature Methods.
Single-Cell and CRISPR
While the human genome was sequenced 20 years ago, we still don’t know the cellular function of most genes. Single-cell CRISPR screens are a great way to cluster genetic perturbations by phenotypes like differentiation, chromosomal instability, the cell cycle, retrovirus activation, alternative-polyadenylation, etc., not to mention the potential for combining scRNA-seq with other phenotypes like imaging or protein measurements. By understanding mechanisms, it should become easier to rationally target multiple genetic dependencies in cancer.
Spatial Transcriptomics
Spatial single-cell transcriptomics is the next wave after single-cell analysis and will be particularly useful to labs studying human disease. Spatial transcriptomics, is the Nature’s 2020 “Method of the Year” and it can be performed on tissue sections using barcode arrays that record the coordinates of mRNA molecules in a sample. This technology was first applied in prostate cancer studies.
Spatial techniques can be divided into those that involve gene expression analysis on micro dissected tissues and those that involve in situ hybridization, in situ sequencing, in situ capturing, and computational reconstruction of spatial data.
Single-molecule fluorescence in situ hybridization (smFISH) is “the beginning of the hybridization-based approaches” with spatial techniques. In this method, multiple oligonucleotides carry fluorescent labels and bind to an RNA molecule. smFISH yields a quantitative mRNA readout with “a near 100% detection sensitivity”.
Single-Cell proteomics
Recent studies have used high-sensitivity mass spectrometry to achieve single-cell proteomics, and another report has coupled click chemistry with mass spectrometry to study lipid metabolism in single cells. Thus, it will soon be possible to study cell signaling pathways and altered metabolism in single cells.
Single-cell technologies are providing unique insights into disease biology and treatment response.
The “cost per cell” currently remains prohibitive for routine analysis. However, it is to be expected that these costs will fall with time as they did for bulk sequencing, allowing this technology to eventually be used in routine patient care. Perhaps it does seem impossible, overwhelming, or ambitious to talk of these numbers, but that’s what was said about the human genome project over 20 years ago.
Pushing single-cell sequencing into clinical application is one of the important missions for clinical and translational medicine (CTM), although there still are a large number of challenges to be overcome.
References
- 1. Single‐cell RNA sequencing technologies and applications: A brief overview, Dragomirka Jovic et.al, Clin Transl Med, 2022 Mar,12
- 2. Single-cell genomics to understand disease pathogenesis: Seitaro Nomura J Hum Genet, 2021, 66, 75-84
- 3. Method of the Year: Spatially resolved transcriptomics, Nature Methods, 2021, 18(1)
- 4. Method of the Year 2019: Single-cell multimodal omics, Nature Methods, 2020,17(1)
#Single-Cell Sequencing Technologies, #genomic DNA sequences, #single‐cell RNA expression, #Single-cell genomics, #Single-cell Transcriptomics, #Single-Cell Epigenomics, #Single-Cell multiomics, #Single-Cell and CRISPR, #Spatial Transcriptomics, #Single-cell proteomics
 US
US IN
IN

