By Archana Deshpande, QA Manager, MedGenome Inc
Introduction
Next-generation sequencing (NGS) data is being increasingly used in clinical diagnosis to identify genetic variation that can be a cause for the disease. A major challenge in using NGS data in a clinical setting is to make the right interpretation because of its huge size and complexity. Also, there are possibilities of technical errors during the sample processing and/or sequencing stage that may be inherent to the kind of sequencing technology used. Therefore, the use of reference standards is of paramount importance to mitigate and minimize these errors.
Reference standards play an important role in the life cycle of a typical NGS method implementation before clinical application. A typical NGS assay life cycle includes assay development, optimization, validation, and continuous quality management – standard is of consequence in all these aspects ranging from assay validation to technical validation to sample processing. This article will discuss the general selection of these reference standards and describe in detail the results of technical validation of a target-capture assay (Illumina’s TruSight Oncology 500 panel) that was performed at MedGenome Labs.
NIST and GIAB Standards
There are several consortiums like GIAB (Genome in a Bottle) and companies (Horizon Diagnostics and SeraCare) that have developed DNA reference material over the years to support clinical translation of whole genome sequencing. NIST (National Institute for Standards and Technology) also had a program to develop whole human genome reference materials. In general, reference standards are well-characterized samples, that are consistent and stable over time. Essentially, the DNA reference is characterized by collating data from various sequencing and bioinformatics methods and from multiple datasets to yield highly confident genotype calls. This data can then be used by laboratories for evaluating assay performance and accreditation agencies for benchmarking results.
In addition to being homogeneous and stable, NIST has defined standards with values that they have certified indicating confidence in their accuracy. This certification indicates that NIST has fully investigated and accounted for all known or suspected sources of bias seen in the data.
MedGenome Validation Data
At MedGenome Labs, we have validated Illumina’s TSO 500 workflow and pipeline using reference samples from SeraCare. TSO 500 is a target-capture based panel that interrogates multiple biomarkers and tumor types; it identifies all relevant DNA and RNA variants implicated in various solid tumor types. Thus, it allows for in-house comprehensive genomic profiling of tumor samples. It also accurately measures key current immuno-oncology biomarkers: microsatellite instability (MSI) and tumor mutational burden (TMB). The other advantage is that the assay has a ctDNA panel that can be used for liquid biopsies.
The workflow for either TSO 500 is a hybrid capture protocol, and we validated our process by using three control ctDNA (with different allele frequencies 0.1%, 0.5% and Wild Type) from SeraCare. The data generated was from as little as 30 ng of starting input.
We performed analysis of the Somatic mutations with gene list present in Seraseq ctDNA Complete Mutation Mix AF 0.5%, AF 0.1% and WT. The analysis included sensitivity, specificity, positive predictive value, and inter-run comparison. Below are some of the results that we obtained.
Library Quality Report
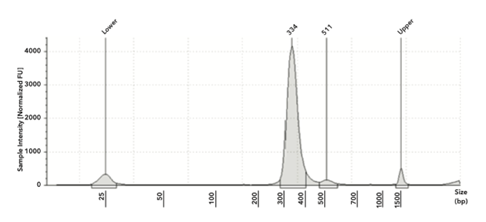
The libraries created from SeraSeq controls ranged from 23 to 46 nM and had an average size of 330 bp with ~200 bp insert size (see Figure 1 for example). This library size fell in the range that is specified in the TSO 500 protocol.
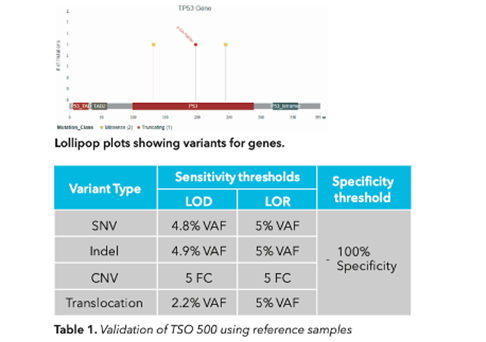
The reference SeraSeq sets were analyzed using TruSight Oncology 500 ctDNA local app. The results were compared with the reference set data provided by the vendor. We were able to obtain 100% sensitivity and specificity for all 3 controls of dataset. The variants are also represented in the lollipop plot which is given below along with the sensitivity and specificity information for the controls (Table 1).
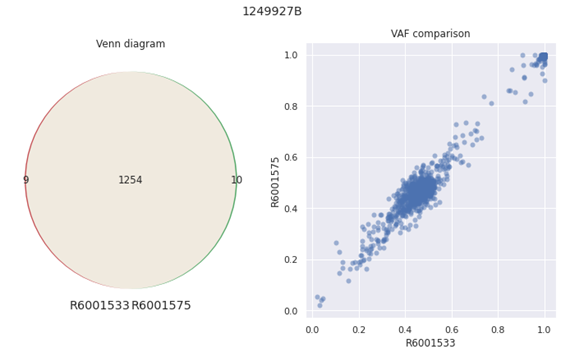
For the inter-run comparison, the samples were compared for the tumor mutation burden count and variant allele frequency (VAF) of the same sample between two runs. The Identified variants were found to be nearly identical and VAF values are highly correlated (See Fig 2 below).
Our process workflow for Illumina’s TSO 500 panel passed the technical validation and we have started offering the TSO500 panel for both solid tumor and ctDNA as part of our services for clients.
Conclusion
DNA reference standards are vital for translational medicine as well as research and MedGenome uses commercially available reference standards to perform technical validation wherever they are available. The validation allows us to have confidence in our process workflows and generated data.
References
-
- 1. Genomic Reference Materials for Clinical Application Justin Zook and Marc Salit Biosystems and Biomaterials Division, National Institute of Standards and Technology, 100 Bureau Dr., Gaithersburg, MD 20899
- 2. Reference standards for next-generation sequencing Simon A. Hardwick, Ira W. Deveson and Tim R. Mercer, Nature Reviews Genetics · June 2017
#NGS Data, #NGS, #benchmark, #standard, #TruSight Oncology, #TSO500, #immuno-oncology biomarkers, #microsatellite instability, #tumor mutational burden
 US
US IN
IN

